Beatrice Boniotti is developing a swine enteroid model! …A what?
In the middle of summer Beatrice Boniotti, and her collaborator Elena Ferrari, sat down with EVA for a discussion around animal viruses that pose veterinary threats and the ongoing work in her lab to establish and characterise a swine enteroid model. « A what ? », you may ask… These organoids, derived from intestinal tissue, offer an alternative to conventional cell culture for studying infection by enteric viruses, host-pathogen interactions and immune response.
But let’s rewind and get to know Beatrice first…
The ice-breaking questions :
-What did you study? Why ? What fascinated you ?
I studied Biological Sciences. I decided to follow this path when I was 15, as Mendel’s laws of inheritance and the mystery of DNA fascinated me! While still at University, I started working on animal viruses and I’m still going.
-What brought you to your current role?
Over the course of my career, I developed additional intersts, such as molecular diagnostics and biobanking. Now I lead a department of more than 35 people.
-One aspect you particularly enjoy in your role?
What I like most about my job is the constant opportunity to become involved in new investigations and always having goals to achieve.
Responsible of the Lab of Molecular Diagnostics and Genetically modified organisms, in the Istituto Zooproffilattico Sperimentale della Lombardia e dell’ Emilia Romagna (IZSLER) (Brescia Italy), Beatrice and her team focus on enteric viruses of zoonotic importance, namely rotaviruses (with a particular interest in infection pathways through cell-surface receptors and the virus’ replication mechanism) and swine viruses, such as porcine reproductive and respiratory syndrome virus (PRRSV) and porcine epidemic diarrhoea virus (PEDV,coronaviridae family). The team aims to develop molecular diagnostics tools for fighting against disease.
What is all the fuss about; some data on current swine health threats.
Production animals represent 40% of the global agriculture production, while they sustain the income and/or livelihood of 1 in 5 people, worldwide. These numbers illustrate the gravity of the burden imposed by animal disease, whose economic impact to date, however, is not fully recorded. Pork, in particular, represents approximately 35% of the meat consumed around the globe, highlighting the potential impact of swine diseases (World Organisation for Animal Health). PRRSV and PEDV are, among other, major reasons for mortality in pigs (1). The epithelium of the small intestine is a complex environment due to its cellular diversity (composed of at least seven cell subtypes) that allows absorption of nutrients and host defence against pathogens (2 and refs therein). In this context, rotaviruses interact with a range of cell-surface receptors (as glycoproteins and gangliosides), therefore managing to infect different kinds of intestinal cells, such as as enterocytes and entero-endocrine cells. The factors that mediate this selectivity, however, remain unknown (2). From a practical point of view, PEDV, targeting the enterocytes of the intestinal epithelium, continues to cause outbreaks in farms across Asia and the US. Stark differences in the infectivity of in-vitro cultured cells have been observed between the EU- versus the US-circulating PEDV strain, hindering the in-vitro study of the infection mechanisms. Conventional cultured cell lines are known to become genotypically modified over time, while they are not able to generate a full immune response upon infection. On the other hand, explants are high maintenance and not very resilient over time (3). Can we work around these limitations? When studying enteric viruses, the need for a functional model on which in-vitro infection and host immune response can be studied is critical.
Enteroids have revolutionised gastrointestinal virus infection studies…a little bit of history.
It all started in 2007 with a discovery in the group of Dr. Hans Clevers, where researchers identified a molecular marker (Lgr5) for stem cells in the intestinal epithelium of mice (4). After establishing that multicellular intestinal epithelial cultures could be grown ex-vivo from isolated Lgr5+ cells, they moved on to develop human intestinal enteroids (5). Meaning : three-dimensional in-vitro cultures derived from proliferating stem cells from crypts, isolated from human gastrointestinal tract biopsies or surgical specimens (6 and refs therein).
In short, enteroids are intestinal organoids that offer a close approximation of the intestinal physiology. Derived either from intestinal tissue or from induced pluripotent stem cells, enteroids are grown in matrigel and develop a 3D structure resembling that of the intestine. The variety of epithelial cells, found in the native tissue, is preserved (absorptive enterocytes, mucus-producing Goblet cells, antimicrobial peptide-producing Paneth cells, hormonesecreting enteroendocrine cells, chemosensory tuft cells, antigen-sampling microfold cells and multipotent proliferative stem cells). Upon disruption of the enteroid 3D structure, the pool of intestinal epithelial cells can be grown in a 2D monolayer culture (3). Overall, the features of the native intestinal epithelium (donor genetics, segmental specification, cell polarization, nutrient and ion transport, barrier function, mucus secretion, and microbicidal peptide production) are maintained in enteroids. This allows the study of different virus internalisation pathways (different enteric viruses infect different kinds of intestinal cells), the host immune response mechanisms (3), testing the efficiency of virus inactivation methods, evaluating the neutralising capacity of human monoclonal antibodies against viruses, assessing the levels of serum neutralising antibodies (7).
What can we use enteroids for?
One of the objectives in Beatrice’s team is to generate functional swine enteroids using isolated crypts from the jejunum (the part of the intestine that is the physiological target of rotavirus and PEDV infection). For the enteroid model to be established, the presence of all intestinal cells (assessed using relevant molecular markers) is verified by RT-PCR, while protein expression is monitored by immunofluorescence. Isolated enteric viruses can then be used to infect the enteroid model, which is grown as a 2D monolayer in order to overcome difficulties linked to access of the 3D model by the virus. This allows the study of the first stages of infection, during which the virus crosses the epithelium to reach secondary sites of infection, leading to more serious pathologies (2). Interestingly, findings generated from the swine model are transferable to humans, due to similarities in the anatomy and physiology of the two, as well as the increasing availability of data on the swine species, at the genomic, transcriptomic and proteomic level (8).
The cellular diversity of the intestinal epithelium, represented in enteroids, is critical for the host immune response to enteric viruses. The ability of enteroids to generate an interferon response (Type III) is linked to the antiviral response(7). Non-epithelial cells (macrophages, neutrophils) can be co-cultured allowing the study of the innate immune response upon infection (3). This can be of particular interest, as different strategies are deployed by immune and intestinal epithelial cells (Type I vs Type III interferon response[s1] , respectively) for neutralising the virus (7). Infection occurs through virus-specific pathways and relative virus permissiveness may vary (2). The antiviral signalling generated by the intestine is also virus-specific: its efficiency depends on the virus’ life cycle and the stage of replication at which the virus has to face the hosts defence mechanism.
…And what is the bigger picture?
Enteroids are not of interest only due to their use in infection studies. As these organoids maintain the genetic identity and the segmental specificity of the donor, they are aligned with the developing idea of personalised medicine (3). As an example, drug therapies for diarrhoeal disease can be tested in these physiologically active models allowing for a tailor-made treatment(9).Within the same lines, the epithelial response to infection of genetically diverse individuals can be followed in culture in-vitro (9). Aside from enteric viruses, SARS-CoV2, the coronavirus we all know too well, is also a candidate for infecting enteroids. SARS-CoV2 replicates not only in the respiratory, but also in the gastrointestinal tract, making enteroids a good model for studying the gastrointestinal implications of the famous virus! And enteroids are only an example. The organoid field is evolving (lung and cardiac organoids are being developed), and will soon be offering promising models for future research.
Text by Semeli Platsaki, PhD
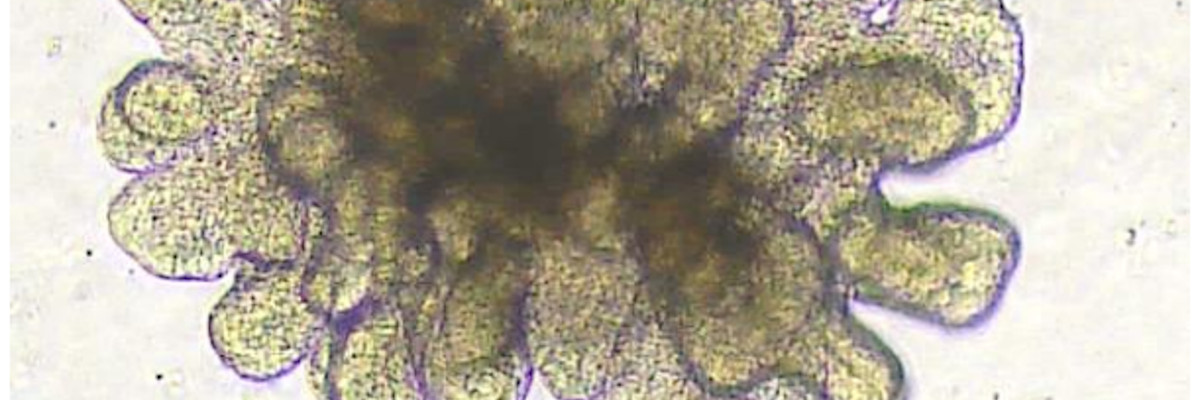
Legend for the enteroid image:
Porcine 3D intestinal organoid, originating from a 7days old piglet. Intestinal portion: jejunum. Cell culture passage n°2, complete media (IntestiCult OGM Mouse Kit – STEMCELL technologies), 4 days growth.
References
1. Gao Q., Zheng Z., Wang H., Yi S., Zhang G., and Gong L. 2021. The new porcine epidemic diarrhea virus outbreak may mean that existing commercial vaccines are not enough to fully protect against the epidemic strains. Front. In Vet. Sci. doi.org/10.3389/fvets.2021.697839
2. Drummond C. G., Bolock A. M., Congrong M., Luke C. J., Good M., and Coyne C. B.,2017.Enteroviruses infect human enteroids and induce antiviral signaling in a cell lineage-specific manner PNAS 114 (7): 1672–1677 https://www.pnas.org/doi/full/10.1073/pnas.1617363114
3. Ranganathan S., Smith E. M., Foulke-Abel J. D., and Barry E. M. 2020. Research in a time of enteroids and organoids: how the human gut model has transformed the study of enteric bacterial pathogens. Gut Microbes. 12 (1) doi.org/10.1080/19490976.2020.1795389
4. Sato T., Vries R. G., Snippert H. J., van deWetering M., Barker N., Stange D. E., van Es J. H., Abo A., Kujala P., Peters P. J., Clevers H. 2009. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 459 (7244): 262–265. DOI: 10.1038/nature07935 [PubMed: 19329995]
5. Sato T., Clevers H. 2013. Growing self-organizing mini-guts from a single intestinal stem cell: mechanism and applications. Science. 340 (6137): 1190–1194. DOI: 10.1126/science.1234852 [PubMed: 23744940]
6. Zou W. Y., Blutt S. E., Crawford S. E., Ettayebi K., Zeng X-L., Saxena K., Ramani S., Karandikar U. C., Zachos N. C., and Estes M. K. 2019. Human intestinal enteroids: new models to study gastrointestinal virus infections. Methods Mol Biol. 1576: 229–247. doi:10.1007/7651_2017_1
7. Nolan L. S. and Baldridge M. T. 2022. Advances in understanding interferon-mediated immune responses to enteric viruses in intestinal organoids. Frontiers in Immunology https://doi.org/10.3389/fimmu.2022.943334
8.Bassols A., CostaC., Eckersall P. D., OsadaJ., SabriàJ., Tibau J. 2014. The pig as an animal model for human pathologies: a proteomics perspective. Proteomics Clinical Applications https://doi.org/10.1002/prca.201300099
9. Saxena K., Blutt S. E., Ettayebi K. …. and Estes M. K. 2016. Human intestinal enteroids: a new model to study human rotavirus infection, host restriction, and pathophysiology. J. of Virology, 90 (1) : 43-56 https://doi.org/10.1128/JVI.01930-15